1,3-diaxial interactions#
Investigate steric interactions in cyclohexane derivatives.
Tasks#
Create and optimize the geometry of a cyclohexane molecule.
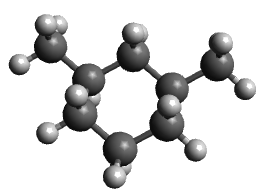
Add methyl substituents to the 1 and 3 positions in a diequatorial arrangement, optimize the geometry and note the energy.
Delete the diequatorial substituents to return to cyclohexane, and optimize the geometry again.
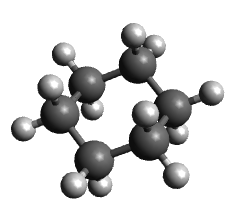
Place methyl substituents in 1,3-diaxial positions, optimize the geometry and note the movement of the groups and the energy.
Solution#
|
|
|
|
|---|---|---|---|
Molecule |
Cyclohexane |
Diequatorial |
Diaxial |
Energy |
30 kJ/mol |
40 kJ/mol |
98 kJ/mol |