Organic Hybridization#
Illustrate the geometry and bond lengths in sp3, sp2 and sp hybridized carbons.
Task#
For each of ethane, ethylene and ethyne, build the molecule and optimize the geometry. Use the measurement tool to examine bond lengths and bond angles.
Solution#
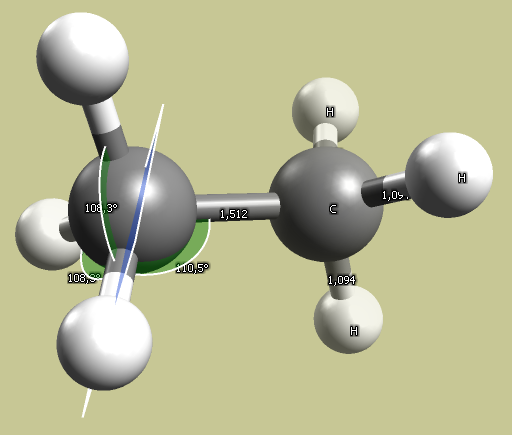
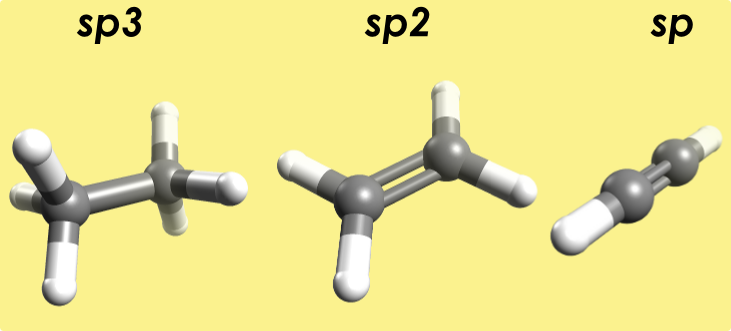
Results using the MMFF94 forcefield:
sp3 - ethane bond angle: 110°; C-C bond length: 1.5 Å.
sp2 - ethylene bond angle: 121°; C-C bond length: 1.336 Å.
sp - ethyne bond angle: 180°; C-C bond length: 1.200 Å.
