Markovnikov’s Rule#
Investigate the stability of reactions using Markovnikov’s rule.
Task#
Build a propene molecule and optimize its geometry. Compare the relative energy of bromine-hydrogen substitution at either the 1-carbon or 2-carbon of the carbon-carbon double bond. Which of these two bromopropane compounds will be the major product of a propene bromination reaction?

Solution#
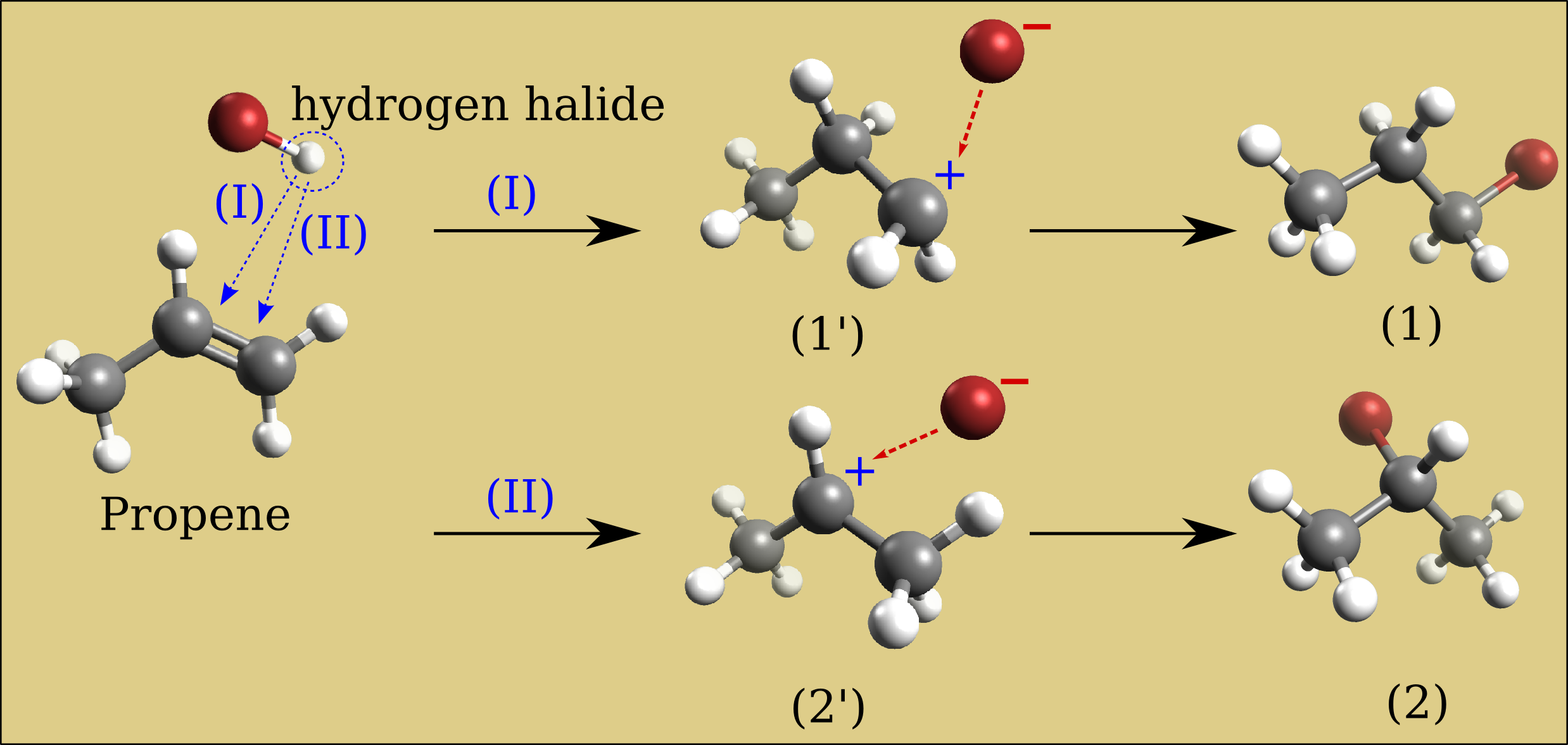
Results of relative energies expressed in kJ/mol (need confirmation in literature).
Force field |
Intermediate primary carbocation (1’) |
Intermediate secondary carbocation (2’) |
1-bromopropane (1) |
2-bromopropane (2) |
|---|---|---|---|---|
Ghemical |
-2.24 |
-2.06 |
-2.39 |
-1.87 |
MMFF94 |
-9.88 |
-3.84 |
-8.96 |
+2.70 |
UFF |
+4.05 |
+3.63 |
+4.15 |
+4.86 |
UFF data is considered in this case, the secondary carbocation intermediate requires less energy (2’) than the primary carbocation intermediate (1’) (resp. 3.6 vs 4 kJ/mol). So compound (2) will be the major form. This is called the Markovnikov’s rule: “the major product of the addition of HX (where X is some atom more electronegative than H, the bromine in our case) to an alkene (here the propene) has the hydrogen atom in the less substituted position and X in the more substituted position”. Mechanisms which avoid the carbocation intermediate such as the presence of dialkyl peroxides will reverse the reaction result and compound (1) becomes the major product (Anti-Markovnikov rule).
